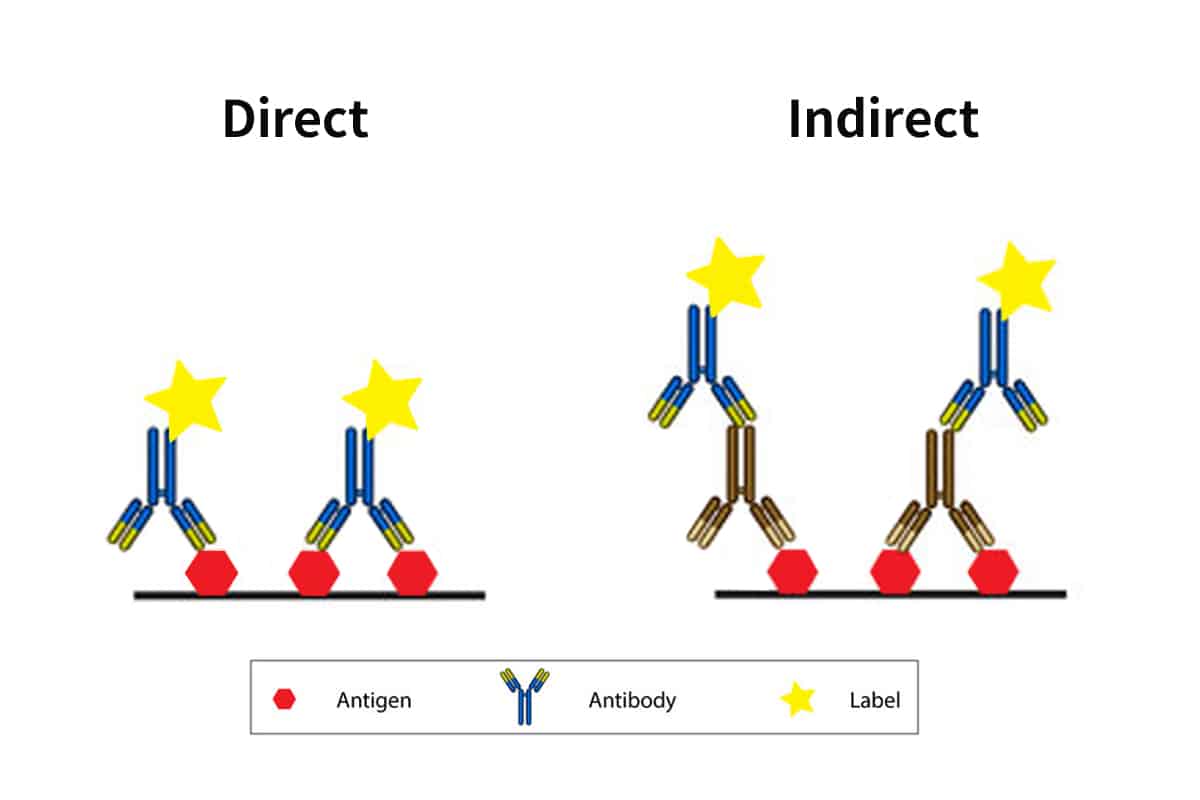
Direct ELISA Vs Indirect ELISA
Direct ELISA Vs Indirect ELISA
Direct ELISA Introduction
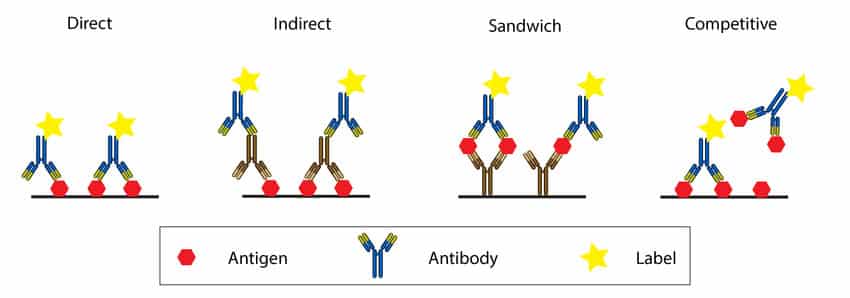
Direct ELISA is the most basic format, requiring only an antigen and an enzyme-conjugated antibody specific to the antigen, and is used to quantify and detect a specific substance (analyte). An antibody or antigen might be used as the analyte. The presence of an antigen in blood serum is determined by how it binds to certain antibodies.
A direct ELISA (enzyme-linked immunosorbent assay) is a plate-based immunosorbent assay used to detect and quantify a specific analyte (e.g., antigens, antibodies, proteins, hormones, peptides, etc.) in a complicated biological sample. Direct ELISA is the simplest and quickest to perform of the four distinct ELISA types.
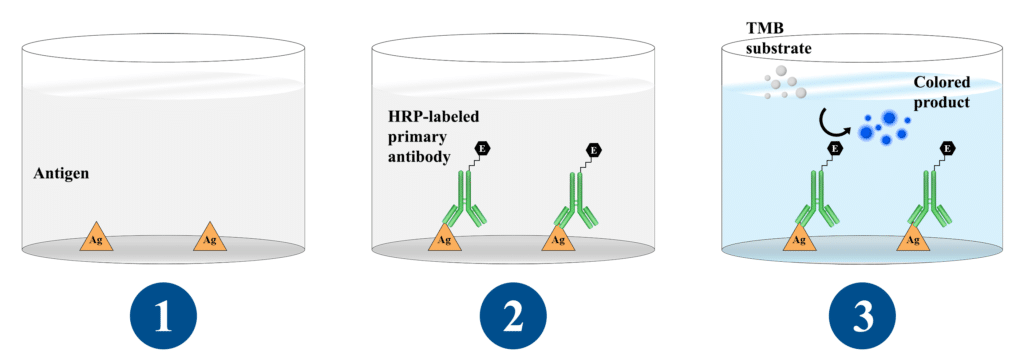
Direct ELISA Advantages
Simple and quick – Only one antibody is needed, and the workflow is only a few steps long. Because fewer stages are necessary, it is much faster than other ELISA procedures.
Less prone to errors – The most evident advantage of direct Elisa over indirect Elisa is that just one antibody is required, and the experiment takes less time to complete. The second advantage is that the secondary antibody’s potential for non-specific cross-reactivity is fully reduced.
Direct ELISA Disadvantages
Less adaptable – Since each target protein requires a unique conjugated primary antibody.
Low sensitivity – There is no signal amplification step, hence assay sensitivity is low.
Increased background noise – Because the method of immobilizing the antigen is not specific, there may be more background noise than with indirect ELISA. This is due to the fact that all proteins in the sample, including the target protein, bind to the plate.
Time-consuming and costly – Labeling primary antibodies for each ELISA system is time-consuming and costly.
Indirect ELISA Introduction
Indirect ELISA is a detection technique that employs a two-step process that involves the binding of a primary antibody and a tagged secondary antibody. A primary antibody specific for the antigen binds to the target, and a secondary antibody labeled against the primary antibody’s host species binds to the primary antibody for detection. However, this may result in nonspecific results due to cross-reactions caused by the secondary antibody.
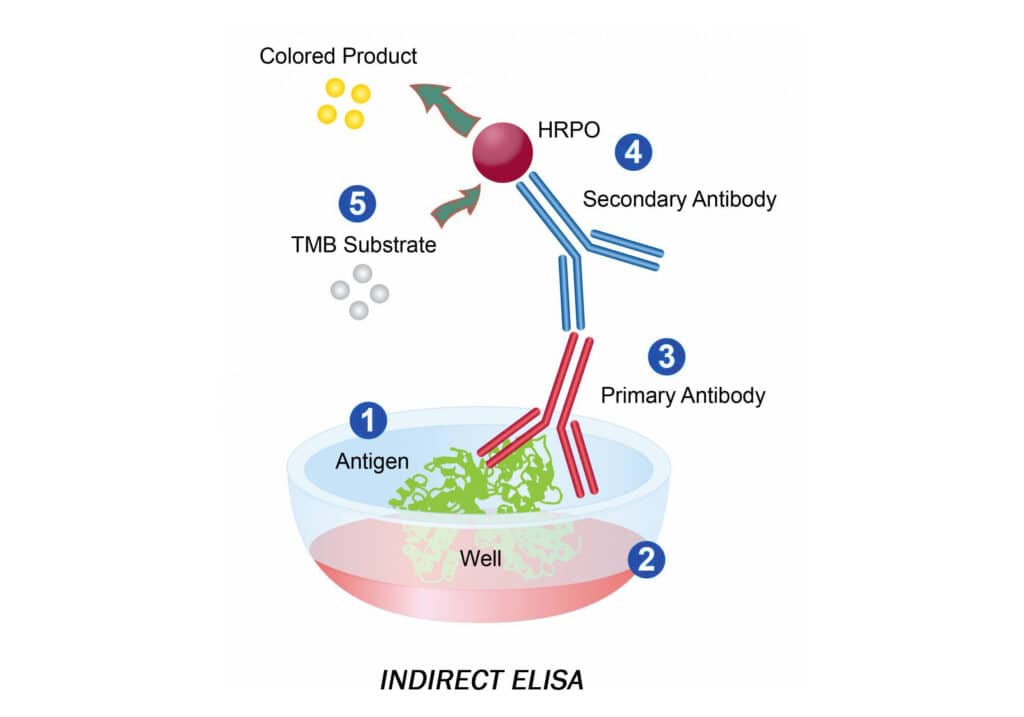
Indirect ELISA Advantages
Sensitivity – Because each primary antibody has numerous epitopes, more than one tagged antibody can bind the primary antibody. No label interacts with the locations on the main antibody, resulting in maximum immunoreactivity. Cost-effective since fewer tagged antibodies are needed.
Greater adaptability – Various primary antibodies can be employed with a single secondary antibody that has been tagged. Additionally, a huge selection of labeled secondary antibodies are offered commercially.
Versatile – One species can produce a variety of primary antibodies, and the same secondary antibody with a label can be employed for detection. Because it is not tagged, the main antibody retains its maximum immunoreactivity.
Cost-effective – Less labeled antibodies are required.
Indirect ELISA Disadvantages
Background noise – Since each target protein requires a unique conjugated primary antibody.
Longer process than a direct ELISA – Since an additional incubation stage is necessary.
Key differences in ELISA
The main distinction between direct and indirect ELISA is the use of one antibody in direct ELISA as opposed to two antibodies in indirect ELISA.
Since only one antibody is required, direct ELISA is quicker to complete and more economical because the antibody is immediately attached to the detecting enzyme. However, the presence of an enzyme may compromise an antibody’s reactivity. Additionally, for every ELISA assay, an enzyme-labeled antibody that is particular to the target antigen should be generated.
Two antibodies are needed for indirect ELISA: an enzyme-linked secondary antibody that is complementary to the primary antibody and a primary antibody for the target antigen. Even though the secondary antibody takes longer and costs more money, it gives you additional options for tagged secondary antibodies and enzyme-substrate detection methods. As long as a suitable primary-secondary antibody combination is present, the labeled secondary antibody can be applied to several ELISA studies.
Meanwhile, the ELISA methodology can be adjusted and improved by using various enzyme-substrate detection systems for the same primary antibody. Additionally, a stronger signal can be displayed than with direct ELISA since the main antibody has more binding sites for secondary antibodies than the antigen does for an antibody.
ELISA Selection Summary
| ELISA Types | Advantages | Disadvantage |
| Direct ELISA | Saves time and reagents using a brief protocol.No secondary antibody cross-reactivity. | All proteins in the sample bind to the surface, which could indicate a high background.No amplification of the signal.High requirement for conjugation of the main antibody. |
| Indirect ELISA | Multiple secondary antibodies will bind to the primary antibody, thereby amplifying the signal.High adaptability: many primary antibodies can be used with the same secondary antibody. | Long protocol when compared to direct ELISA.Cross-reactivity risk with secondary antibody. |
| Sandwich ELISA | High specificity: entails the detection of two distinct epitopes on the same antigen by two antibodies.suited for sophisticated samples.High sensitivity and adaptability: both direct and indirect procedures are possible. | Demanding design: Finding two antibodies that are directed against the same target but recognize different epitopes and function well together can occasionally be difficult. |
| Competitive ELISA | It is determined by the base ELISA used.Small antigens are suitable. | It is determined by the base ELISA used. |