Hydrogel Introduction
Hydrogel Introduction
What Is Hydrogel?
Hydrogel is a crosslinked hydrophilic polymer that does not dissolve in water. They are highly absorbent yet maintain well-defined structures. These properties underpin several applications, especially in the biomedical area. Many hydrogels are synthetic, but some are derived from nature.
Hydrogel is a kind of extremely hydrophilic three-dimensional network structure gel, which swells rapidly in water and can hold a large volume of water without dissolving in this swollen state.
Due to the presence of the cross-linked network, the hydrogel can swell and retain a large amount of water, and the amount of water absorbed is closely related to the degree of cross-linking. The higher the degree of crosslinking, the lower the water absorption. This property is much like a soft tissue. The water content in a hydrogel can be as low as a few percent or as high as 99 percent. The aggregated state of the gel is neither completely solid nor completely liquid. The behavior of solids is that they can maintain a certain shape and volume under certain conditions, and the behavior of liquids is that solutes can diffuse or penetrate from the hydrogel.
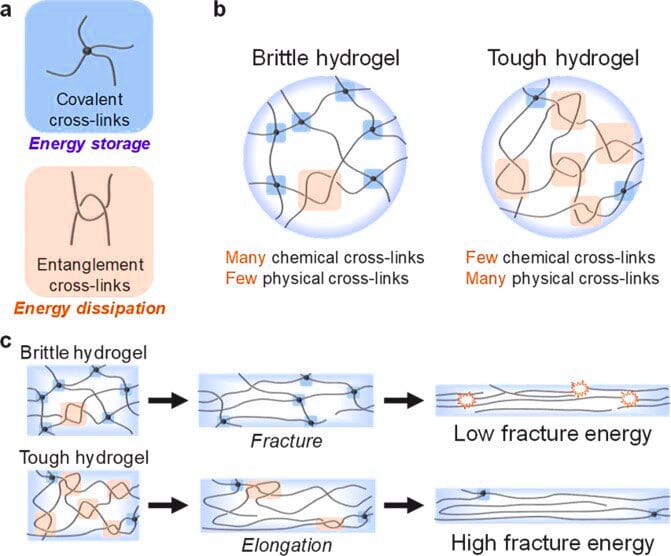
The crosslinks which bond the polymers of a hydrogel fall under two general categories: physical and chemical. Chemical hydrogels have covalent cross-linking bonds, whereas physical hydrogels have non-covalent bonds. Chemical hydrogels result in strong irreversible gels due to the covalent bonding, and they may also possess harmful properties which makes them unfavourable for medical applications. Physical hydrogels on the other hand have high biocompatibility, aren’t toxic, and are also easily reversible, by simply changing an external stimulus such as pH or temperature; thus they are favourable for use in medical applications. Physical crosslinks consist of hydrogen bonds, hydrophobic interactions, and chain entanglements (among others). A hydrogel generated through the use of physical crosslinks is sometimes called a ‘reversible’ hydrogel. Chemical crosslinks consist of covalent bonds between polymer strands. Hydrogels generated in this manner are sometimes called ‘permanent’ hydrogels.
Research History of Hydrogel
In a paper published on January 9, 2013, Johns Hopkins University in the United States said that they have developed a new type of hydrogel biomaterial that can be injected into small holes in bones during cartilage repair surgery to help stimulate the patient’s marrow to produce stem cells to grow new cartilage. In clinical trials, the new cartilage coverage rate reached 86%, and postoperative pain was also greatly reduced.
Erich also said that the research team is developing the next generation of grafted materials, one of which is a hydrogel and a binder, which will be integrated into one material. Additionally, they are working on techniques to lubricate joints and reduce inflammation.
The latest research in Canada shows that hydrogel (Hydrogel) is not only beneficial to stem cell transplantation, but also accelerates the repair of eye and nerve damage. The research team pointed out that the jelly-like hydrogel is an ideal medium for stem cell transplantation, which can help stem cells survive in the body and repair damaged tissues.
Zhou Feng, a researcher at the Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, used molecular engineering to design ultra-high-strength hydrogels with double-crosslinked networks, which greatly improved the mechanical properties of the hydrogels.
Hydrogel for Myocardial Infarction
Myocardial infarction (Myocardial Infarction) is one of the largest causes of death in the global population. It is characterized by myocardial necrosis caused by acute or persistent ischemia and hypoxia, and the myocardium itself lacks the ability to regenerate for repair.
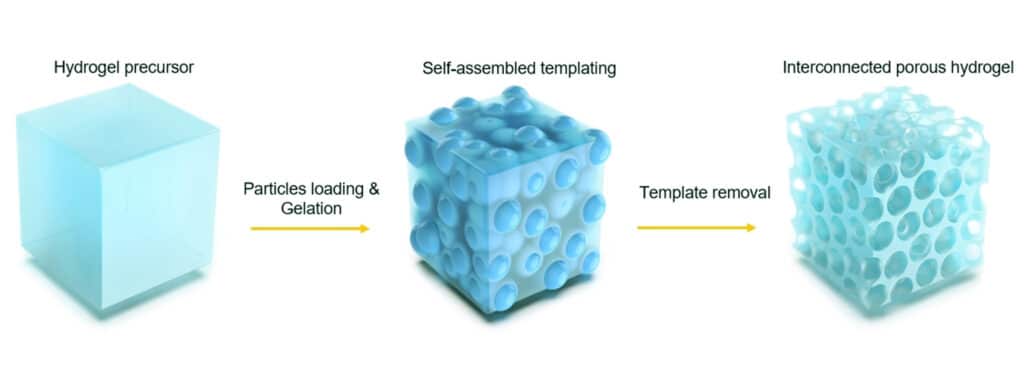
Applying a suspension of stem cells to the infarct site to differentiate into cardiomyocytes appears to be the most intuitive method. However, it is difficult for these free single cells to remain at the infarct site, and most of them “escape” into the blood circulation within a few days. If stem cells are encapsulated in a porous small tissue scaffold of hydrogel and then implanted into the infarcted site, although the survival will be improved, the rate of oxygen diffusion in the scaffold is often difficult to meet the needs of stem cells, and the latter will die in these scaffolds in large numbers. . In addition, the site of myocardial infarction tends to accumulate a considerable number of macrophages and their secreted cytokines. This will not only directly damage the implanted stem cells, but also be further stimulated by the addition of the latter, which is very detrimental to the survival of stem cells.
Having said that, stem cell therapy for myocardial infarction is easier said than done. However, even so, recently, the research team of Professor Liu Zhenguo, He Xiaoming and Noah Weisleder of Ohio State University has still explored a set of effective stem cell therapy, which has achieved remarkable results in a mouse model of myocardial infarction.
The method mimics the process of early embryonic development. The researchers first implanted more than 20 mouse embryonic stem cells into a microcapsule encased in a semipermeable alginate hydrogel shell, which resembles the zona pellucida of an early embryo. These stem cells first divide, forming aggregates of more than 1,000 stem cells, entering a stage similar to a morula. After that, the researchers initially induced the stem cell clusters in the microcapsules into cardiomyocytes in the early differentiation stage by introducing transcription factors such as BMP-4 and bFGF for 3 days, and then dissolved the shell of the microcapsules with sodium citrate, The initially differentiated cell clusters described above are released. These cell clusters, in turn, are encapsulated into alginate-chitosan (ACM) micromatrix by immersing in chitosan and oxidized alginate solutions successively to form another microcapsule for myocardial preparation implant. It is worth pointing out that despite repeated wrapping and re-wrapping processes, the cell clusters described above maintained a high degree of viability.
Smearing Hydrogel
In recent years, the rise of tumor immunotherapy has brought new hope to the majority of cancer patients. Among them, CAR-T cell therapy is undoubtedly of concern. In simple terms, this cancer therapy is to collect T cells from cancer patients and reprogram them, and then deliver them back to the patient. These modified CAR-T cells will precisely destroy cancer cells, allowing cancer patients reborn.
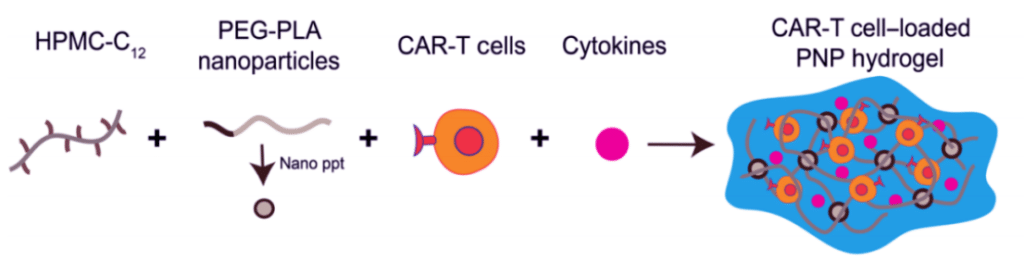
On April 8, 2022, researchers from Stanford University published a research paper entitled: Delivery of CAR-T cells in a transient injectable stimulatory hydrogel niche improves treatment of solid tumors in the journal Science Advances.
The study develops a simple and efficient method of CAR-T delivery: CAR-T cells and cytokines are added to a specially formulated hydrogel, which provides a temporary, immune cell-activated in vivo environment, and then pumped to activated CAR-T cells to attack the tumor tissue.
More importantly, this delivery method has the potential to treat distant tumors, which opens new doors for CAR-T therapy in solid tumors.