Interleukin
Interleukin
introduction
Interleukins (ILs) are a group of cytokines (secreted proteins and signal molecules) that were first seen to be expressed by white blood cells (leukocytes). ILs can be divided into four major groups based on distinguishing structural features. However, their amino acid sequence similarity is rather weak (typically 15–25% identity). The human genome encodes more than 50 interleukins and related proteins.
The name “interleukin” was chosen in 1979, to replace the various different names used by different research groups to designate interleukin 1 (lymphocyte activating factor, mitogenic protein, T-cell replacing factor III, B-cell activating factor, B-cell differentiation factor, and “Heidikine”) and interleukin 2 (TSF, etc.). This decision was taken during the Second International Lymphokine Workshop in Switzerland (27-31 May 1979 in Ermatingen). The term interleukin derives from (inter-) “as a means of communication”, and (-leukin) “deriving from the fact that many of these proteins are produced by leukocytes and act on leukocytes”. The name is something of a relic; it has since been found that interleukins are produced by a wide variety of body cells. The term was coined by Dr Vern Paetkau, University of Victoria.
Some interleukins are classified as lymphokines, lymphocyte-produced cytokines that mediate immune responses
Interleukin families
Interleukin 1
Interleukin 1 alpha and interleukin 1 beta (IL1 alpha and IL1 beta) are cytokines that participate in the regulation of immune responses, inflammatory reactions, and hematopoiesis. Two types of IL-1 receptor, each with three extracellular immunoglobulin (Ig)-like domains, limited sequence similarity (28%) and different pharmacological characteristics have been cloned from mouse and human cell lines: these have been termed type I and type II receptors.
Interleukin 2
T lymphocytes regulate the growth and differentiation of T cells and certain B cells through the release of secreted protein factors. These factors, which include interleukin 2 (IL2), are secreted by lectin- or antigen-stimulated T cells, and have various physiological effects. IL2 is a lymphokine that induces the proliferation of responsive T cells. In addition, it acts on some B cells, via receptor-specific binding, as a growth factor and antibody production stimulant. The protein is secreted as a single glycosylated polypeptide, and cleavage of a signal sequence is required for its activity.
Interleukin 3
Interleukin 3 (IL3) is a cytokine that regulates hematopoiesis by controlling the production, differentiation and function of granulocytes and macrophages. The protein, which exists in vivo as a monomer, is produced in activated T cells and mast cells, and is activated by the cleavage of an N-terminal signal sequence.
Interleukin 4
Interleukin 4(IL4) is produced by CD4+ T cells specialized in providing help to B cells to proliferate and to undergo class switch recombination and somatic hypermutation. Th2 cells, through production of IL-4, have an important function in B-cell responses that involve class switch recombination to the IgG1 and IgE isotypes.
Interleukin 5
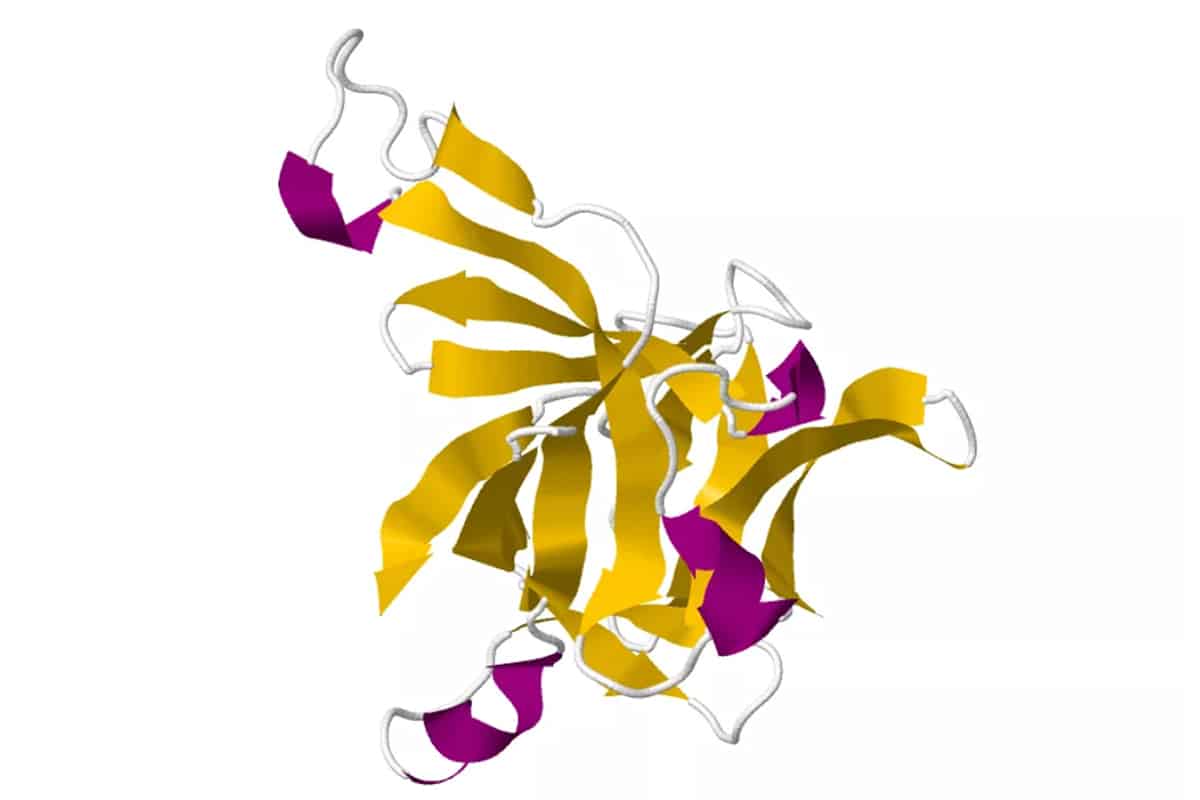
Interleukin 5 (IL5), also known as eosinophil differentiation factor (EDF), is a lineage-specific cytokine for eosinophilpoiesis. It regulates eosinophil growth and activation, and thus plays an important role in diseases associated with increased levels of eosinophils, including asthma. IL5 has a similar overall fold to other cytokines (e.g., IL2, IL4 and GCSF), but while these exist as monomeric structures, IL5 is a homodimer. The fold contains an anti-parallel 4-alpha-helix bundle with a left handed twist, connected by a 2-stranded anti-parallel beta-sheet.
Interleukin 6
Interleukin 6 (IL6), also referred to as B-cell stimulatory factor-2 (BSF-2) and interferon beta-2, is a cytokine involved in a wide variety of biological functions. It plays an essential role in the final differentiation of B cells into immunoglobulin-secreting cells, as well as inducing myeloma/plasmacytoma growth, nerve cell differentiation, and, in hepatocytes, acute-phase reactants.
Interleukin 7
Interleukin 7 (IL-7) is a cytokine that serves as a growth factor for early lymphoid cells of both B- and T-cell lineages.
Interleukin 8
Interleukin 8 is a chemokine produced by macrophages and other cell types such as epithelial cells, airway smooth muscle cells and endothelial cells. Endothelial cells store IL-8 in their storage vesicles, the Weibel-Palade bodies. In humans, the interleukin-8 protein is encoded by the CXCL8 gene. IL-8 is initially produced as a precursor peptide of 99 amino acids, which then undergoes cleavage to create several active IL-8 isoforms. In culture, a 72 amino acid peptide is the major form secreted by macrophages.
Interleukin 9
Interleukin 9 (IL-9) is a cytokine that supports IL-2 independent and IL-4 independent growth of helper T cells. Early studies had indicated that Interleukin 9 and 7 seem to be evolutionary related and Pfam, InterPro and PROSITE entries exist for interleukin 7/interleukin 9 family. However, a recent study has shown that IL-9 is, in fact, much closer to both IL-2 and IL-15, than to IL-7.
Interleukin 10
Interleukin 10 (IL-10) is a protein that inhibits the synthesis of a number of cytokines, including IFN-gamma, IL-2, IL-3, TNF, and GM-CSF produced by activated macrophages and by helper T cells. In structure, IL-10 is a protein of about 160 amino acids that contains four conserved cysteines involved in disulphide bonds.
Interleukin 11
Interleukin 11 (IL-11) is a secreted protein that stimulates megakaryocytopoiesis, initially thought to lead to an increased production of platelets (it has since been shown to be redundant to normal platelet formation), as well as activating osteoclasts, inhibiting epithelial cell proliferation and apoptosis, and inhibiting macrophage mediator production. These functions may be particularly important in mediating the hematopoietic, osseous and mucosal protective effects of interleukin 11.
Interleukin 12
Interleukin 12 (IL-12) is a disulphide-bonded heterodimer consisting of a 35kDa alpha subunit and a 40kDa beta subunit. It is involved in the stimulation and maintenance of Th1 cellular immune responses, including the normal host defence against various intracellular pathogens, such as Leishmania, Toxoplasma, Measles virus, and Human immunodeficiency virus 1 (HIV). IL-12 also has an important role in enhancing the cytotoxic function of NK cells and role in pathological Th1 responses, such as in inflammatory bowel disease and multiple sclerosis.
Interleukin 13
Interleukin 13 (IL-13) is a pleiotropic cytokine that may be important in the regulation of the inflammatory and immune responses. It inhibits inflammatory cytokine production and synergises with IL-2 in regulating interferon-gamma synthesis. The sequences of IL-4 and IL-13 are distantly related.
Interleukin 15
Interleukin 15 (IL-15) is a cytokine that possesses a variety of biological functions, including stimulation and maintenance of cellular immune responses. IL-15 stimulates the proliferation of T lymphocytes, which requires interaction of IL-15 with IL-15R alpha and components of IL-2R, including IL-2R beta and IL-2R gamma (common gamma chain, γc), but not IL-2R alpha.
Interleukin 17
IL-17 is a potent proinflammatory cytokine produced by activated memory T cells. This cytokine is characterized by its proinflammatory properties, role in recruiting neutrophils, and importance in innate and adaptive immunity. Not only does IL-17 play a key role in inflammation of many autoimmune diseases, such as RA, allergies, asthma, psoriasis, and more, but it also plays a key role in the pathogenesis of these diseases