Leukemia Inhibitory Factor
Leukemia Inhibitory Factor
introduction
Leukemia inhibitory factor, or LIF, is an interleukin 6 class cytokine that affects cell growth by inhibiting differentiation. When LIF levels drop, the cells differentiate. LIF derives its name from its ability to induce the terminal differentiation of myeloid leukemic cells,thus preventing their continued growth. Other properties attributed to the cytokine include: the growth promotion and cell differentiation of different types of target cells, influence on bone metabolism, cachexia, neural development, embryogenesis and inflammation.p53 regulated LIF has been shown to facilitate implantation in the mouse model and possibly in humans. It has been suggested that recombinant human LIF might help to improve the implantation rate in women with unexplained infertility. LIF is normally expressed in the trophectoderm of the developing embryo, with its receptor LIFR expressed throughout the inner cell mass. As embryonic stem cells are derived from the inner cell mass at the blastocyst stage, removing them from the inner cell mass also removes their source of LIF. Recombinant LIF has been produced in plants by InVitria.
How was LIF discovered?
It has been 33 years since LIF was discovered and cloned. Some cytokines that regulate hematopoietic cell proliferation and differentiation have been discovered in the 1880s, such as granulocyte colony-stimulating factor (G-CSF), macrophage colony-stimulating factor (M-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), Multi-CSF (IL-3) and Interferon-γ (IFN-γ) etc. The common feature of these cytokines is that they can not only promote the differentiation of hematopoietic cells, but also stimulate their proliferation. That is to say, while promoting the differentiation of leukemia cells, these cytokines will also greatly increase the number of leukemia cells, which may aggravate the condition of leukemia instead of treating leukemia. Therefore, scientists still need to find a cytokine that only induces the differentiation of leukemia cells, but does not promote the proliferation of leukemia cells, to treat leukemia.
What is the structure of LIF?
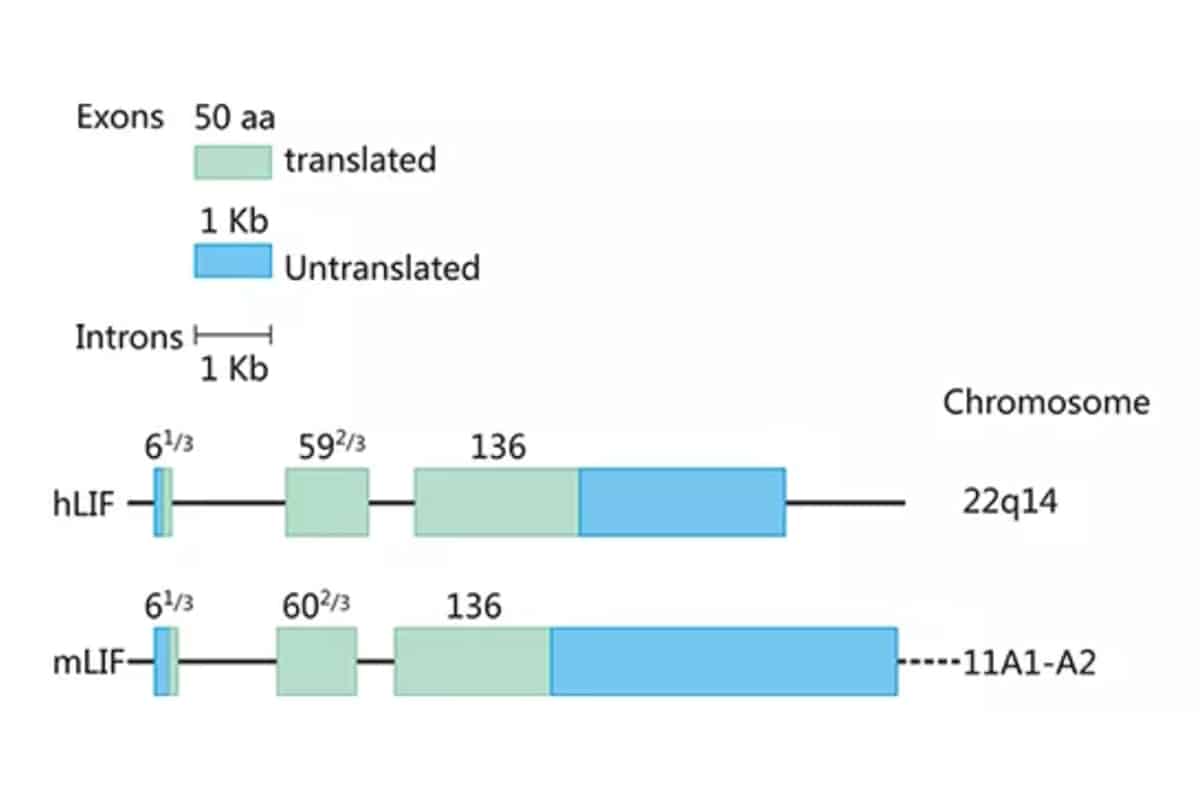
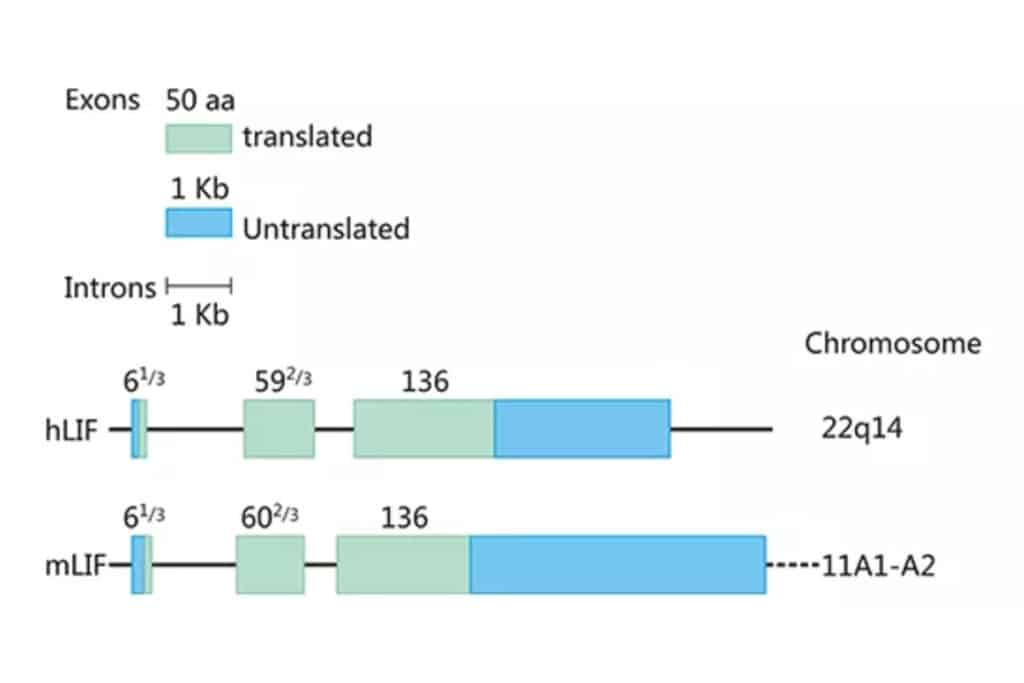
As shown in Figure 1, the human and mouse LIF genes are located on chromosomes 22q14 and 11A1-A2, respectively, with lengths of 6.0 and 6.3 kb, containing three exons of 61/3, 592/3, and 136 amino acids, the coding region sequence is highly conserved, and the homology is 78-94%. Intramolecular disulfide bonds play an important role in maintaining the structure and biological activity of LIF molecules. The molecular weight and charge of LIF vary according to the degree of glycosylation, with a molecular weight of 38-64 kDa and an IP of 8.6-9.2. The biological function of LIF in vitro appears to be independent of the degree of glycosylation, but whether glycosylation affects LIF stability and function in vivo remains to be determined. Human and mouse LIF have 78% homology at the amino acid level, and human LIF has similar activity on mouse-derived cells, while mouse LIF has a weaker effect on human cells.
How does LIF function biologically?
LIF can be expressed in different cells, including activated T cells, monocytes, glial cells, liver fibroblasts, bone marrow stromal cells, embryonic stem cells, thymic epithelial cells, and many others. The complex of LIF and LIFRβ will further non-covalently bind to gpl30 to form a heterogeneous receptor complex with high affinity (Figure 2). The formation of a high-affinity complex of LIF, LIFRβ and gpl30 is required for signal transduction in most cell types. The heterodimeric receptor complex itself has no intrinsic tyrosine kinase activity.
-1024x545.png)
In conclude, LIF is a widely expressed cytokine with distinct glycosylation patterns. Moreover, LIF receptors are present in many different tissues, including liver, central nervous system, macrophages, bone, kidney, placenta, the ICM of embryos, and the uterine epithelium. LIF can phosphorylate STAT3 through several reactions. Then, phosphorylated STAT3 dimers and translocates into the nucleus and activates target gene transcription. LIF-JAK/STAT3 is a well-established signaling pathway in pancreatic ductal adenocarcinoma (PDAC), CRC, ovarian cancer, and non-small lung cell carcinoma (NSCLC). In addition, LIF has also been shown to activate other signaling pathways, such as YAP/TEAD and AKT/mTOR.
Reference:
- GRCh38: Ensembl release 89: ENSG00000128342 – Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000034394 – Ensembl, May 2017
- Human PubMed Reference:”. National Center for Biotechnology Information, U.S. National Library of Medicine.
- Mouse PubMed Reference:”. National Center for Biotechnology Information, U.S. National Library of Medicine.